Everlywell IPO: COVID-19 Test Company to Go Public?
The thought of an Everlywell IPO has investors on the edge of their seats. That’s because the company launched an at-home coronavirus test.
Since this is the first step to mass testing, investors want in on the action. Currently, there isn’t any publicly traded Everlywell stock. But is it possible that will change?
Here’s what we know…
Everlywell IPO: The Business
Julia Cheek founded Everlywell in 2015 and is CEO today. Everlywell is an at-home health test company based in Austin, Texas. It provides at-home lab testing kits and digital results within days. The company offers 35 kits for tests ranging from food sensitivity to STDs to fertility.
In 2017, Cheek went on the TV show Shark Tank. There, she made a deal with shark Lori Greiner. The offer was a $1 million line of credit with an 8% interest rate and a 5% equity stake to Greiner.
Over the years, Everlywell registered a 300% increase in customers year over year. The company has partnerships with CVS, Target and Humana.
And now Everlywell has a new product out to test for COVID-19.
Cheek said:
Everlywell was founded to give people affordable, convenient access to lab testing. Never has our mission been more important. Our team has been working around the clock with top scientists and laboratories in the nation to develop a test that we will make available at the lowest price possible while covering our costs, at no profit to the company.
Currently, the company is private. But if its coronavirus kit launches efficiently, investors hope an Everlywell IPO can follow.
There are a lot of questions surrounding the issue. For now, you might be wondering…
Who Can Get the Coronavirus Test?
There is a global shortage of supplies and tests for the coronavirus. Many press conferences and government meetings have centered on this issue. And people quarantined in their homes consider it a big concern. Many people fear exposure and the chance they could be carriers. But despite this big leap forward, not everyone can get the at-home test.
On March 23, Everlywell released its first batch of 30,000 kits. However, the night before, the company made an announcement. It was limiting the kits to healthcare companies and professionals, to test workers on the front lines of the outbreak. Everlywell claimed it received requests from hospitals, doctors, nurses and healthcare providers, along with a plea from the White House.
Cheek commented:
It has become increasingly apparent there is a desperate need for healthcare workers caring for sick patients on the front lines to have priority access to testing for COVID-19. There has been a massive concern by these professionals who are being exposed, and could contract the virus, but there aren’t enough tests to see if they have it themselves.
But people can soon apply for a test. In order to receive one, you must go to Everlywell’s website and fill out some questions. Like all tests, the COVID-19 test needs a doctor’s approval to take. The questions are about basic health, symptoms and risk factors for the coronavirus disease. Then a telemedicine doctor from PWNHealth will review the answers. They’ll determine if the person is qualified for testing, according to the CDC. PWNHealth, a national network of physicians, prescribes diagnostic tests and is a partner to Everlywell.
Cheek has stated she hopes the company can produce up to 250,000 kits a week. But even at that rate, it would take over a year to produce enough kits to test the entire U.S. population.
But supplies are limited. There aren’t enough nasal swabs to create tests for everyone. The biggest manufacturer of these is a company in Italy, which is a strictly quarantined country. That’s why kits come with one swab and are limited to one kit per household.
So Everlywell’s goal of 250,000 tests a week seems a little far from reality. And if the company can’t keep up with production demand, then a possible Everlywell IPO wouldn’t be as likely to succeed.
The next questions to ask is…
How Do You Use the Coronavirus Test Kit?
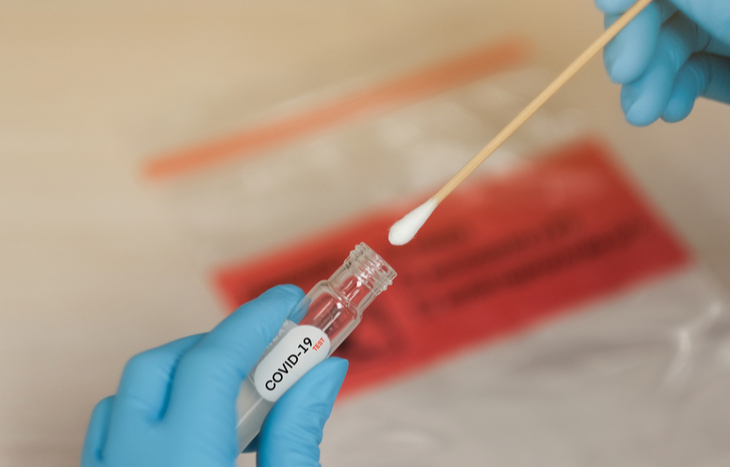
Once you’re approved to receive the test, you can choose overnight shipping (additional $30) or standard two-day shipping. Then, Everlywell has you register your kit online to track your data. The kit includes two samplings: a nasal swab and saliva sample.
There are concerns that at-home testers won’t use the long nasal swab properly. Failure to do so can result in false negatives. And Dr. George Rutherford, a physician researching the control of infectious diseases at the University of California commented:
This is not just swabbing the first quarter-inch of the nose: you have to dig in there. If you are going to do it, I’d say that make sure getting the specimen hurts and your eyes water. Give yourself a thorough swabbing. Once the samples are taken, wipe down the package with the provided alcohol disinfectant pad. Each kit comes with a prepaid shipping label to mail the test to a certified lab partner.
On March 8, Everlywell announced a $1 million development incentive. The company enlisted eight medical labs across the country that will split the money. All labs comply with the Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA).
Test results should be available online within 48 hours. Free telehealth consultations will be available for people who receive positive results.
But when thinking of an Everlywell IPO, a big question is…
What Does the COVID-19 Test Cost?
Currently, the test costs $135. However, this provides no profit for the company. The cost is as low as possible, covering the production, testing and analysis process costs.
With that in mind, there’s some good news.
First, you can use participating health savings accounts and flexible spending accounts.
Secondly, Cheek says the company has reached out to government and public health officials to see how Everlywell can provide free tests. Healthcare providers who have received tests for workers are covering the cost and giving them to employees at no charge.
If the government can help create free tests, that’s great for people at home who need to be tested as well as investors. With the backing of the federal government, Everlywell could become a powerhouse on the market, pending an Everlywell IPO.
Is Everlywell’s COVID-19 Test Worth Taking?
People are skeptical about taking a test at home. But there’s a good chance Everlywell’s test is accurate and reliable. The COVID-19 test adheres to the FDA’s EUA guidelines. And it follows the same process as COVID-19 tests being used in hospitals.
Also, when it comes to people 65 years of age and older, Cheek makes a good point:
This option is especially useful for people who want to avoid potential exposure at healthcare facilities or are quarantined at home because they are experiencing symptoms.
As everyone tries to stop the spread of coronavirus by quarantining and social distancing, Everlywell’s test might be the best chance society has so far. It will help people with the virus get medical attention and keep them from spreading the disease.
Now, let’s look at the question investors are asking…
Will Everlywell IPO?
The company has made no moves to go public. However, the world is facing a situation it’s never faced before. And Everlywell is stepping up to the challenge.
This development in coronavirus testing puts Everlywell in a unique situation. It has provided an answer to a stressful question: How can everyone get tested? Assuming the company can deliver on its lofty goals, the demand for Everlywell stock will likely increase.
If you’re looking for investment opportunities, Investment U is the place to be. Sign up for our free e-letter below to receive investment tips and trends researched by our experts. There’s something for everyone, whether you’re a beginner or experienced investor.
If Everlywell decides to take advantage of the investor demand, the company could go public once the coronavirus is contained. However, it depends on when that happens, the markets, and the successful production and delivery of the product. One thing is for sure. Don’t count an Everlywell IPO out.
[adzerk-get-ad zone="245143" size="4"]About Amber Deter
Amber Deter has researched and written about initial public offerings (IPOs) over the last few years. After starting her college career studying accounting and business, Amber decided to focus on her love of writing. Now she’s able to bring that experience to Investment U readers by providing in-depth research on IPO and investing opportunities.





